
Institute of Biochemistry and Pathobiochemistry
Molecular Cell Biology
E-Mail
Tel.: +49-(0)234-32-28428
The Winklhofer lab focuses on mechanisms underlying neurodegenerative diseases on the molecular, cellular and systems level. A major topic is the role of the ubiquitin system in maintaining neuronal proteostasis and integrity. Another focus are mitochondria as key organelles in orchestrating stress response and adaptive pathways as well as interorganellar communication at the interface of neuroprotective and innate immune signaling.
To address these research interests experimentally, we are using state-of-the-art techniques in cell biology, molecular biology, protein biochemistry, and advanced light microscopy, including super-resolution microscopy and live cell imaging.
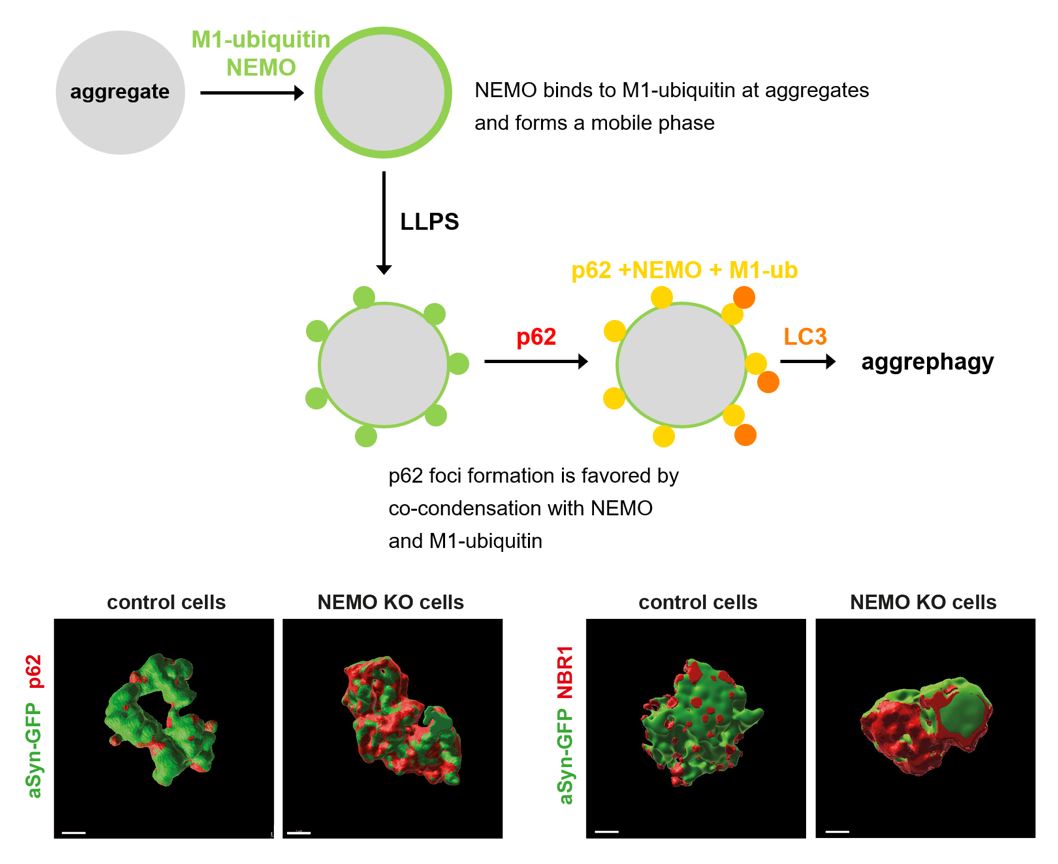
NEMO is a ubiquitin-binding protein implicated in canonical NF-κB pathway activation in innate immune signaling, cell death regulation and host-pathogen interactions. In this study, we identified a yet unknown function of NEMO in proteostasis regulation by promoting autophagosomal clearance of protein aggregates. NEMO-deficient cells accumulate misfolded proteins upon proteotoxic stress and are vulnerable to proteostasis challenges. Moreover, a patient with a mutation in the NEMO-encoding IKBKG gene resulting in defective binding of NEMO to linear ubiquitin chains, developed a widespread mixed brain proteinopathy, including α-synuclein, tau and TDP-43 pathology. NEMO amplifies linear ubiquitylation at α-synuclein aggregates and promotes the local concentration of p62 into foci. In vitro, NEMO lowers the threshold concentrations required for ubiquitin-dependent phase transition of p62. In summary, NEMO reshapes the aggregate surface for efficient autophagosomal clearance by providing a mobile phase at the aggregate interphase that favors co-condensation with p62.
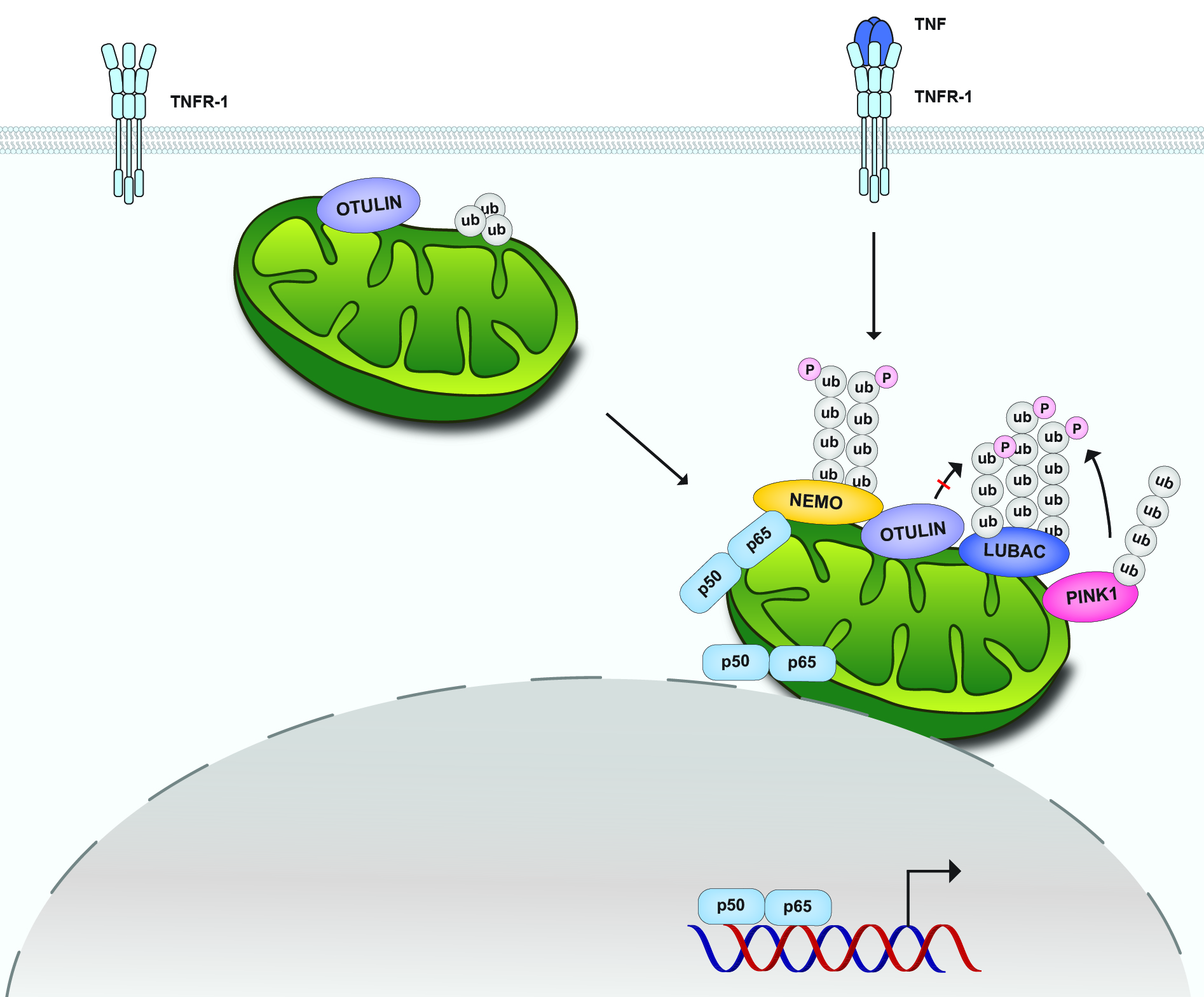
Mitochondria are increasingly recognized as cellular hubs to orchestrate signaling pathways that regulate metabolism, redox homeostasis, and cell fate decisions. Recent research revealed a role of mitochondria also in innate immune signaling, however, the mechanisms of how mitochondria affect signal transduction are poorly understood. Our work revealed that the NF-ĸB pathway activated by TNF employs mitochondria as a platform for signal amplification and shuttling of activated NF-ĸB to the nucleus. TNF treatment induces the recruitment of HOIP, the catalytic component of the linear ubiquitin chain assembly complex (LUBAC), and its substrate NEMO to the outer mitochondrial membrane, where M1- and K63-linked ubiquitin chains are generated. NF-ĸB is locally activated and transported to the nucleus by mitochondria, leading to an increase in mitochondria-nucleus contact sites in a HOIP-dependent manner. Notably, TNF-induced stabilization of the mitochondrial kinase PINK1 contributes to signal amplification by antagonizing the M1-ubiquitin-specific deubiquitinase OTULIN.
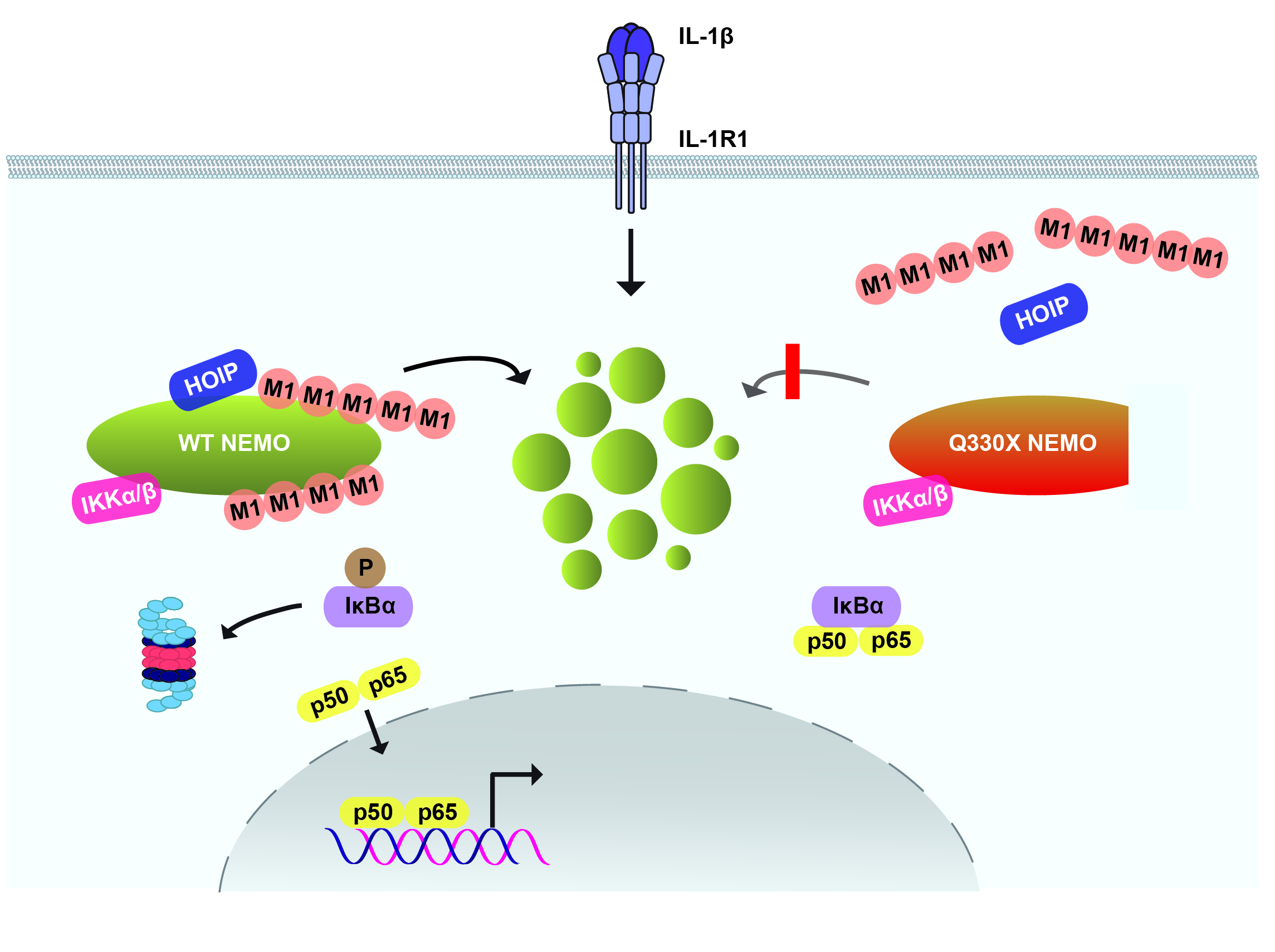
The NF-κB essential modulator NEMO is the core regulatory component of the inhibitor of κB kinase (IKK) complex, which is a critical checkpoint in canonical NF-κB signaling downstream of innate and adaptive immune receptors. In response to various stimuli, such as tumor necrosis factor (TNF) or interleukin-1β (IL-1β), NEMO binds to linear or M1-linked ubiquitin chains generated by LUBAC, promoting its oligomerization and subsequent activation of the associated kinases. We observed that M1-ubiquitin chains induce phase separation of NEMO and the formation of NEMO assemblies in cells after exposure to IL-1β. Phase separation is promoted by both binding of NEMO to linear ubiquitin chains and covalent linkage of M1-ubiquitin to NEMO and is essential but not sufficient for its phase separation. Supporting the functional relevance of NEMO phase separation in signaling, a pathogenic NEMO mutant, which is impaired in both binding and linkage to linear ubiquitin chains, does not undergo phase separation and is defective in mediating IL-1β-induced NF-κB activation.
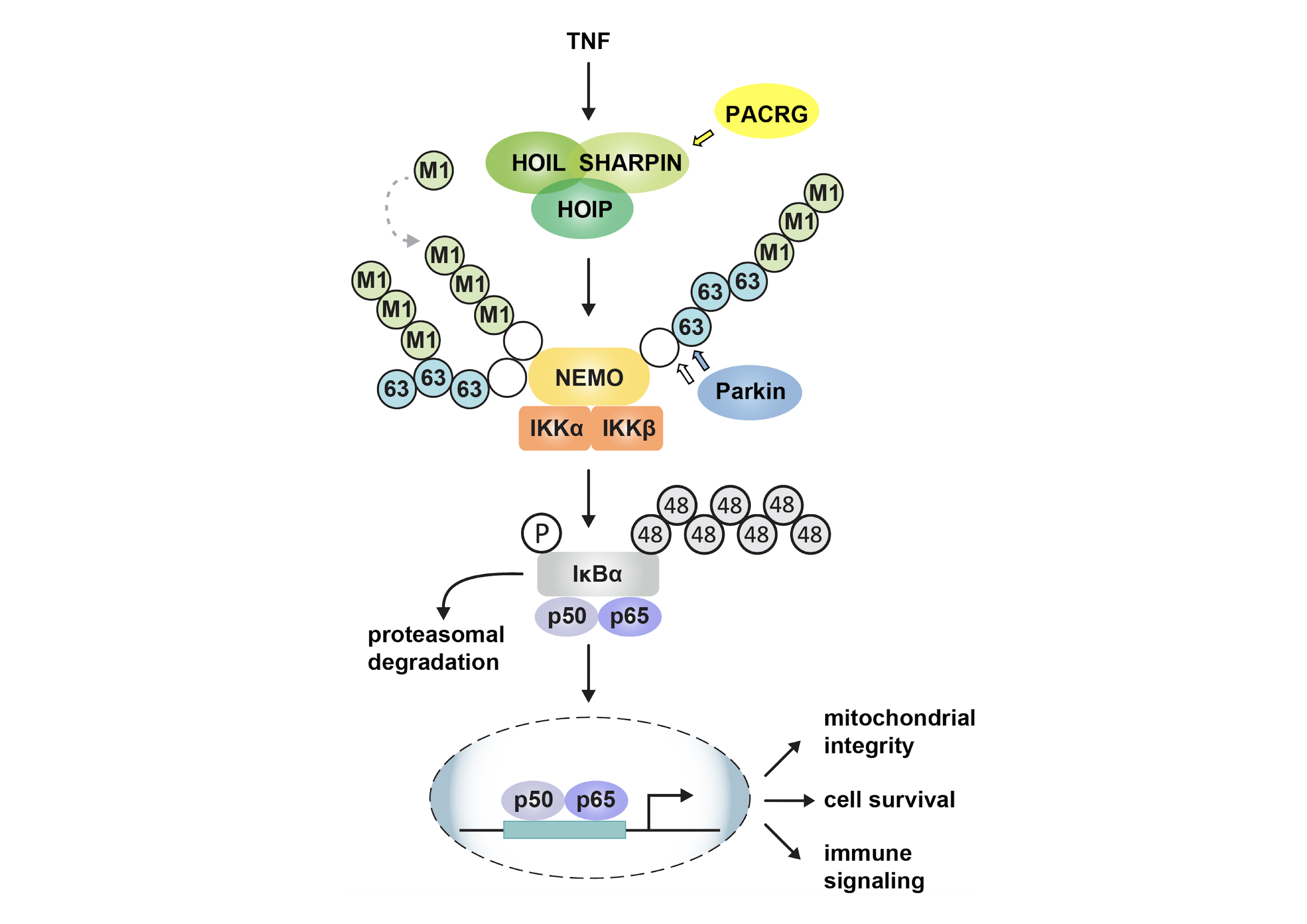
The Parkin coregulated gene (PACRG) shares a bi-directional promoter with Parkin (PRKN), which encodes an E3 ubiquitin ligase. Parkin is important in mitochondrial quality control and protection against stress, we therefore tested whether PACRG also affects these pathways. PACRG does not play a role in mitophagy but does play a role in tumor necrosis factor (TNF) signaling. Similarly to Parkin, PACRG promotes NF-κB activation in response to TNF stimulation. TNF-induced nuclear translocation of the NF-κB subunit p65 and NF-κB-dependent transcription are decreased in PACRG-deficient cells. Defective canonical NF-κB activation in the absence of PACRG is accompanied by a decrease in linear ubiquitylation mediated by the linear ubiquitin chain assembly complex (LUBAC), which is composed of the two E3 ubiquitin ligases HOIP and HOIL-1L and the adaptor protein SHARPIN. Upon TNF stimulation, PACRG is recruited to the activated TNF receptor complex and interacts with LUBAC components. PACRG can functionally replace SHARPIN in this context. In SHARPIN-deficient cells, PACRG prevents LUBAC destabilization, restores HOIP-dependent linear ubiquitylation, and protects cells from TNF-induced apoptosis. This function of PACRG in positively regulating TNF signaling may help to explain the association of PACRG and PRKN polymorphisms with an increased susceptibility to intracellular pathogens.
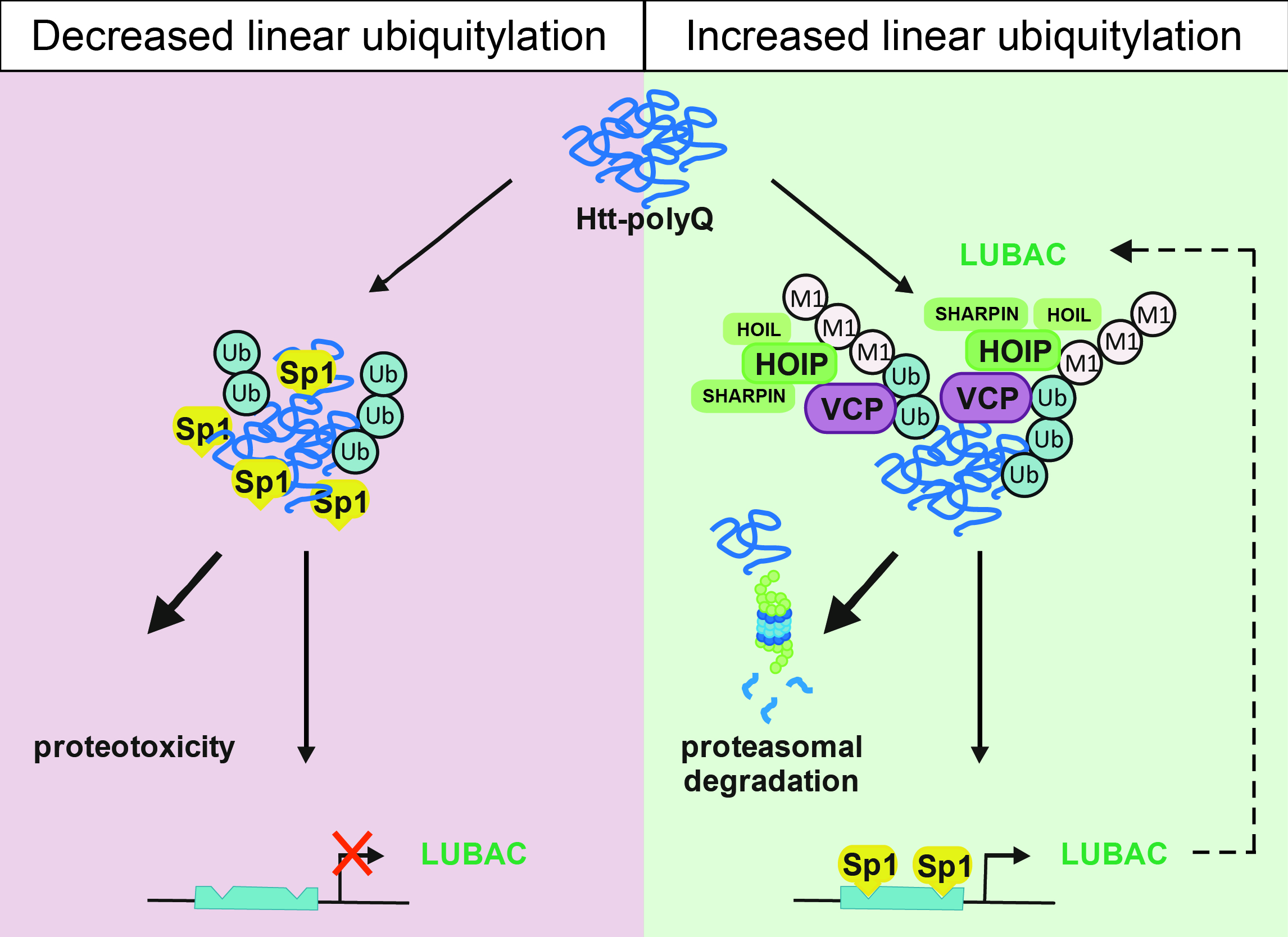
Neurodegenerative diseases are characterized by the accumulation of misfolded proteins in the brain. Insights into protein quality control mechanisms to prevent neuronal dysfunction and cell death are crucial in developing causal therapies. We discovered that various diseases-associated protein aggregates are modified by the linear ubiquitin chain assembly complex (LUBAC). HOIP, the catalytic component of LUBAC is recruited to misfolded Huntingtin in a p97/VCP-dependent manner, resulting in the assembly of linear polyubiquitin. As a consequence, the interactive surface of misfolded Huntingtin species is shielded from unwanted interactions, for example with the low complexity sequence domain-containing transcription factor Sp1, and proteasomal degradation of misfolded Huntingtin is facilitated. Notably, all three core LUBAC components are transcriptionally regulated by Sp1, linking defective LUBAC expression to Huntington's disease. In support of a protective activity of linear ubiquitylation, silencing of OTULIN, a deubiquitinase with unique specificity for linear polyubiquitin, decreases proteotoxicity, whereas silencing of HOIP has the opposite effect. These findings identify linear ubiquitylation as a protein quality control mechanism and hence a novel target for disease-modifying strategies in proteinopathies.